Optimal Medical Therapy with or without PCI for Stable Coronary Disease, William E. Boden, M.D.,and others, NEJM april 12, 2007.
This study was performed to evaluate and compare optimal medical therapy with percutaneous coronary intervention (PCI).
In patients with stable coronary artery disease, it remains unclear whether an initial management strategy of percutaneous coronary intervention (PCI), combined with intensive pharmacologic therapy and lifestyle intervention (Optimal Medical Therapy, OMT) is superior to OMT alone in reducing the risk of cardiovascular events.
The trial involved 2287 patients who had objective evidence of myocardial ischemia and significant coronary artery disease. Between 1999 and 2004, 1149 patients underwent PCI with optimal medical therapy and 1138 received OMT alone.
The outcome studied was death from any cause and non-fatal myocardial infarction during a follow-up period of 2.5 to 7.0 years (median, 4.6).
Treatment
All patients received optimal medical therapy that included:
- Antiplatelet therapy with aspirin or clopidogrel, if aspirin intolerance was present. Patients undergoing PCI received aspirin and clopidogrel.
- Beta blockers and Nitrates in both groups included long acting metoprolol, amlodipine, and isosorbide mononitrate, alone or in combination, along with either lisinopril or losartan as standard secondary prevention.
- All patients received aggressive therapy for Hypercholesterolemia.
In patients undergoing PCI, target-lesion revascularization was always attempted, and complete revascularization was performed as clinically appropriate. Success after PCI as seen on angiography was defined as normal coronary-artery flow and less than 50% stenosis in the luminal diameter after balloon angioplasty and less than 20% after coronary stent implantation, as assessed by visual estimation of the angiograms before and after the procedure. Clinical success was defined as angiographic success plus the absence of inhospital myocardial infarction, emergency CABG, or death. Drug-eluting stents were not approved for clinical use until the final 6 months of the study, so few patients received these intracoronarydevices.
- A total of 2168 patients (95%) had objective evidence of myocardial ischemia and were studied for a median follow-up period was 4.6 years.
- Average age was 60 years, but 85% were male patients, reflecting where the study was performed (VA hospitals).
- All patients had normal heart function (EF:60%)and almost equal distributions of 1-, 2- or 3-vessel CAD.
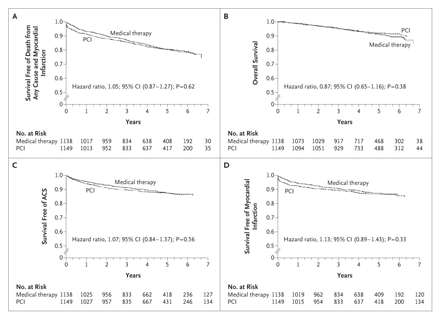
Kaplan–Meier Survival Curves
In Panel A, the estimated 4.6-year rate of the composite primary outcome of death from any cause and nonfatal myocardial infarction was 19.0% in the PCI group and 18.5% in the medical-therapy group. In Panel B, the estimated 4.6-year rate of death from any cause was 7.6% in the PCI group and 8.3% in the medical-therapy group. In Panel C, the estimated 4.6-year rate of hospitalization for acute coronary syndrome (ACS) was 12.4% in the PCI group and 11.8% in the medical-therapy group. In Panel D, the estimated 4.6-year rate of acute myocardial infarction was 13.2% in the PCI group and 12.3% in the medical-therapy group.
Conclusions
As an initial management strategy in patients with stable coronary artery disease, PCI did not reduce the risk of death, myocardial infarction, or other major cardiovascular events when added to optimal medical therapy. Although the addition of PCI to optimal medical therapy reduced the prevalence of angina, it did not reduce long term rates of death, nonfatal myocardial infarction, and hospitalization for acute coronary syndromes.

Comments 3
Pingback: SYNTAX 3 Analysis - Cardiac Health
Pingback: Excessive Stent Use - Cardiac Health
Pingback: Stenting for stable coronary artery disease is wrong! - Cardiac Health