Physicians are often asked to estimate the risk for peri-operative myocardial infarction or cardiac death in patients being considered for non-cardiac surgery. Almost 30 million patients undergo non-cardiac surgery annually in the United States; about one third have known coronary artery disease or risk factors for atherosclerosis. The 500 000 patients who undergo peripheral vascular procedures each year are thought to have a particularly high risk for perioperative cardiac events.
|
Major Cardiac Event Rates by Cardiac Risk Index (3) |
|
|
Class |
Event Rate (95% CI), % |
|
I (0 risk factors) |
0.4 (0.05–1.5) |
|
II (1 risk factor) |
0.9 (0.3–2.1) |
|
III (2 risk factors) |
6.6 (3.9–10.3) |
|
IV (>3 risk factors) |
11.0 (5.8–18.4) |
Functional status can usually be estimated from the ability to perform activities of daily living, and can be expressed as metabolic equivalents (METs); the resting or basal oxygen consumption (VO 2) of a 70kg, 40 years old man in a resting state is 3.5 cc/kg/min, or 1 MET.
Functional capacity has thus been classified as:
|
Excellent |
> 10 METs` |
| Good |
7 – 10 METs |
|
Moderate |
4 – 6 METs |
| Poor |
< 4 METs |
Peri-operative cardiac and long-term risks are increased in patients unable to meet a 4 MET demand during most normal daily activities. In a series of 600 consecutive patients undergoing major non- cardiac procedures, peri-operative myocardial ischemia and cardiovascular events were more common in patients who reported poor exercise tolerance (inability to walk 4 blocks or climb 2 flights of stairs), even after adjustment for baseline characteristics known to be associated with increased risk. The likelihood of a serious complication occurring was inversely related to the number of blocks that could be walked or flights of stairs that could be climbed.
The results of noninvasive testing can be used to determine the need for additional preoperative testing and treatment. In some patients with documented CAD, the risk of coronary intervention or corrective cardiac surgery may approach or even exceed the risk of the proposed noncardiac surgery. This approach may be appropriate, however, if it significantly improves the patient’s long-term prognosis.
THERAPIES TO REDUCE THE RISK FOR PERI-OPERATIVE CARDIAC COMPLICATIONS
Coronary Artery Bypass Grafting
No randomized, controlled trials have assessed the benefit of coronary artery bypass grafting (CABG) before non-cardiac surgery, but patients with previous CABG have low rates of cardiac complications with non-cardiac surgery. Using the Coronary Artery Surgery Study (CASS) registry data, Eagle and colleagues (2002) found that patients who underwent major vascular, abdominal, thoracic, or head and neck surgery after previous CABG had fewer peri-operative deaths and myocardial infarctions than patients receiving medical therapy.
CABG is rarely indicated simply to “get a patient through” noncardiac surgery. In many patients, prophylactic CABG is more likely to harm than benefit most patients undergoing non-cardiac surgery. However, if the patient has symptoms or coronary anatomy that mandate CABG independently of planned non-cardiac surgery, CABG is indicated.
Percutaneous Coronary Intervention
No randomized, controlled trials have shown that percutaneous coronary intervention (PCI) is beneficial as prophylactic therapy in patients undergoing non-cardiac surgery.
In 2000, Kaluza and colleagues (4) suggested caution in performing non-cardiac surgery soon after coronary arterial stenting. He studied 40 patients and found that among those who underwent surgery within two weeks of stent placement the mortality rate was 32%. Among patients who had non-cardiac surgery within 6 weeks of successful stent placement, 20% died, 18% had nonfatal myocardial infarction, and 28% had major bleeding. Percutaneous coronary intervention should be reserved for patients with an acute coronary syndrome or stable angina refractory to medical therapy. If coronary stenting is performed, elective non-cardiac surgery should be deferred for 6 weeks or longer.
Analysis of the Mayo Clinic Percutaneous Coronary Intervention and Surgical databases
between 1990 and 2000 (5) identified 207 patients who underwent surgery within two months following successful coronary stent placement. Eight of 207 patients had major adverse cardiac events. Of these, six patients died, two of whom suffered or were suffering an MI before dying. Their data clearly indicated that the risk of stent thrombosis and other complications is increased when surgery is performed within six weeks after stent placement.
In a recent article by Cruden et al. from the Scottish Coronary Revascularisation Register Steering Committee (6), findings supported current recommendations that noncardiac surgery should be deferredfor at least 6 weeks after bare-metal stent implantation. Furthermore, an increased cardiac risk, although diminishing in magnitude, may extend beyond 6 weeks, irrespective of the type of stent used.
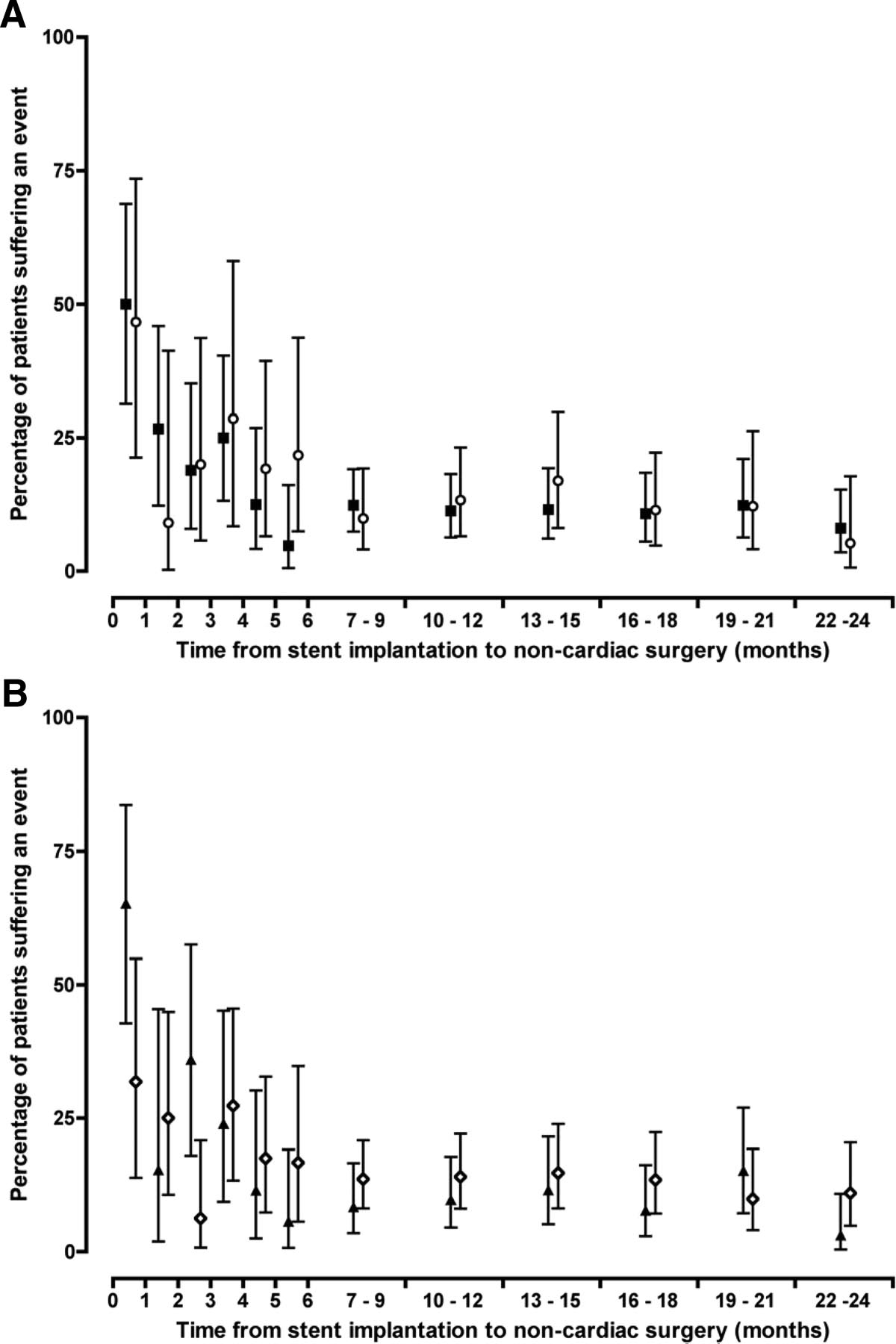
Effect of stent type (A) (bare metal [squares] vs drug eluting [circles]) and initial indication for coronary stent implantation (B) (acute coronary syndrome [triangles] vs stable coronary artery disease [diamonds]) on in-hospital mortality and ischemic cardiac events according to delay from stent implantation to noncardiac surgery. Adverse events occurred more frequently where surgery was performed within 1 month of stent implantation compared with surgery undertaken beyond 1 month (P<0.05 for all; X2 test). Compared with stent implantation for stable coronary disease, patients undergoing surgery within 1 month of coronary stent implantation for an acute coronary syndrome were at greater risk of death or ischemic cardiac events (65% vs 32%; P<0.037; X2 test).
(From: Circ Cardiovasc Interv June 2010)
“Patients undergoing noncardiac surgery after recent coronary stent implantation are at increased risk of perioperative myocardial ischemia, myocardial infarction, and death, particularly after an acute coronary syndrome. For at least 2 years after percutaneous coronary intervention, cardiac outcomes after noncardiac surgery are similar for both drug-eluting and bare-metal stents.”
β Adrenergic Blockade
Since publication of the ACC/AHA and ACP guidelines on risk stratification for non-cardiac surgery, two randomized, controlled trials and one large nonrandomized report have shown that β blocker therapy reduces peri-operative cardiac complications.
Current studies, suggest that appropriately administered beta-blockers reduce perioperative ischemia and may reduce the risk of MI and death in high-risk patients. When possible, beta-blockers should be started days or weeks before elective surgery, with the dose titrated to achieve a resting heart rate between 50 and 60 beats per minute.
Anti Platelet therapy and PCI
Stent thrombosis, a low-frequency event with serious, life-threatening consequences has been observed up to 4 years after implantation. According to the present guidelines, patients with coronary stents are to be treated with dual anti platelet therapy. In case surgery is needed, the risk of a fatal stent thrombosis needs to be balanced against the risk of hemorrhagic complications on continued anti platelet medication.
In a pooled analysis (7) of 4 years of follow-up data of cohorts that included patients treated with sirolimus-eluting stents,as well as others treated with corresponding bare-metal stents, patients with definite or probable stent thrombosis were identified, of whom 31% died and 84% had a myocardial infarction.
If dual anti platelet therapy has to be withdrawn in preparation of a non-cardiac operation, a patient is at a particularly high risk for stent thrombosis, especially within the first 6 weeks after implantation. Abrupt discontinuation of anti platelet therapy can lead to a rebound effect marked by an inflammatory pro thrombotic state, increased platelet adhesion and aggregation, and excessive thromboxane A2 activity. Surgery further increases the pro thrombotic and inflammatory state, which, combined with incompletely endothelialized drug-eluting stents, can lead to stent thrombosis and, consequently, myocardial infarction and/or death.
The US Food and Drug Administration recommends that dual antiplatelet therapy be continued for at least 3 months after placement of a sirolimus-eluting stent and 6 months after placement of a paclitaxel-eluting stent. Recent data suggest, however, that this duration of antiplatelet therapy may not be sufficient and that at least 1 year of therapy may be needed.
For patients with recent drug-eluting stent placement in whom surgery cannot be delayed, dual antiplatelet therapy should be continued without interruption if the stent was placed within the prior 6 months. If the stent was placed more than 6 months before urgent surgery, aspirin should be continued without interruption (at ≥ 81 mg/day) and clopidogrel should be continued until 5 days before surgery and resumed as soon as possible after surgery (at a loading dose of 300 mg followed by 75 mg/day) (8).
CONCLUSIONS
Estimating the risk of a cardiac complication in an individual patient is difficult and complex. Given recent evidence regarding the limited value of coronary revascularization before non-cardiac surgery, the indication for pre-operative testing is limited to the group in whom coronary revascularization may be beneficial, independent of non-cardiac surgery:
- Patients considered at risk for myocardial infarction or cardiac death during non-cardiac surgery should receive a β blocker to reduce the risk for peri-operative cardiac events;
- If the non-cardiac surgery is urgent or emergent, then cardiac risks, the risk of bleeding, and the long- term benefit of coronary revascularization must be weighed, and if coronary revascularization is absolutely necessary, CABG combined with the non-cardiac surgery could be considered;
- Clinical trials have shown that only β blocker therapy reduces the risk contrary to PCI and/or CABG for peri-operative cardiac events except in certain high risk situations where CABG is the superior choice over PCI.
- The frequent use of stents in patients with CAD has given rise to new and complex problems in managing the handling and withdrawal of anti platelet therapy.
References:
- ACC/AHA 2007 Guidelines on Peri-operative Cardiovascular Evaluation and Care for Non-cardiac Surgery, J Am Coll Cardiol, 2007; 50:1591 242,
- ACC/AHA Guideline Update for Perioperative Cardiovascular Evaluation for Noncardiac Surgery—Executive Summary, Eagle et al., JACC Vol. 39, No. 3, February 6, 2002:542–53
- Cardiac Events in Patients Undergoing Non-cardiac Surgery: Shifting the Paradigm from Noninvasive Risk Stratification to Therapy; Grayburn, MD, Ann Intern Med. 2003;138:506-511.
- Kałuza GL, Joseph J, Lee JR, Raizner ME, Raizner AE. Catastrophic outcomes
of noncardiac surgery soon after coronary stenting. J Am Coll Cardiol. 2000;35:1288-94 - Clinical outcome of patients undergoing non-cardiac surgery in the two months following coronary stenting, Stephanie H. Wilson et al., J Am Coll Cardiol, 2003; 42:234-240 (http://content.onlinejacc.org/cgi/reprint/42/2/234.pdf)
- Nicholas L.M. Cruden, PhD, et al., Previous Coronary Stent Implantation and Cardiac Events in Patients Undergoing Noncardiac Surgery, Circulation: Cardiovascular Interventions. 2010 (http://circinterventions.ahajournals.org/cgi/content/abstract/CIRCINTERVENTIONS.109.934703v1)
- Stent Thrombosis in Randomized Clinical Trials of Drug-Eluting Stents , Laura Mauri, M.D., et al., N Engl J Med 2007;356:1020-9.
- Perioperative management of warfarin and antiplatelet therapy, Jaffer, MD, www.ccjm.org/content/76/Suppl_4/S37 (http://www.ccjm.org/content/76/Suppl_4/S37.full.pdf+html)