SURGICAL ANATOMY
Knowledge of detailed cardiac anatomy is a prerequisite for successful surgery. Nowhere is this more important than in the setting of congenital cardiac malformations.—R.H. Anderson, B.R. Wilcox10
In this chapter, the anatomy of the pericardium, heart, and great vessels is reviewed in some detail. Advances in surgical instrumentation, techniques, and medication have facilitated the development of cardiac surgical procedures for repair of congenital cardiac defects, correction of cardiac vascular insufficiency, replacement of diseased cardiac valves, implantation of electronic devices for regulation of pacemaking activity, and replacement of the heart itself. The reader seeking the particulars of surgical procedures relating to the correction of congenital malformations or of pathologic processes should consult appropriate texts that treat thoracic or cardiac surgery in detail.
Pericardium
Topographic Relations
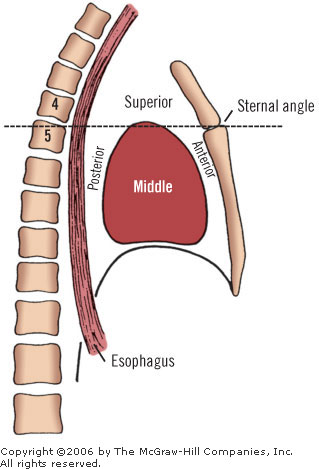
The pericardial sac and the heart within reside in the mediastinum, an area between the pleural sacs. It is bounded anteriorly by the sternum and posteriorly by the thoracic vertebrae.
The mediastinum is divided arbitrarily into superior and inferior portions by a transverse plane passing through the sternal angle of Louis and the T4 intervertebral disk (Fig. 7-1). The inferior mediastinum is further subdivided into anterior, middle and posterior sections. The middle mediastinum is defined by the pericardium and its contents: the heart (Fig. 7-2) and the roots of the eight great vessels (aorta, pulmonary trunk, superior and inferior vena cavae, and four pulmonary veins).
|
||
|
|
||
|
In front of the pericardium are the structures of the anterior mediastinum. These include the first four sternebrae, the lower part of the thymus, and connective tissues. Behind the pericardium are the principal contents of the posterior mediastinum: the aorta, the esophagus, the azygos system, and the fifth through the eighth thoracic vertebrae. Above is the superior mediastinum; below, the diaphragm forms a lower limit for the middle mediastinum.
PERICARDIAL SAC
The pericardial sac, or parietal pericardium, is formed by two layers: an outer fibrous layer and an inner serous layer, responsible for secretion of the fluid film within the pericardial sac (Fig. 7-3). The simple squamous epithelium (mesothelium) that forms the serous lining of the pericardial cavity is a portion of the primitive embryonic celom. Therefore, it is similar to the lining of the pleural and peritoneal cavities. At the points of exit of the vessels from the pericardial sac, the fibrous layer becomes continuous with the adventitia of the vessels and the pretracheal fascia. There the serous lining is also reflected over the surface of the heart, as the visceral pericardium or epicardium. Deep to this layer is a variably thick lamina of connective tissue, which can be thought of as representing the fibrous layer of the pericardial sac.
|
||
|
PERICARDIAL CAVITY
The parietal and visceral layers of pericardium form a closed sac, the pericardial cavity. Two topographic areas within the sac are of special importance. One of these is a part of the pericardial cavity called the oblique sinus. It is found behind the heart, and is bounded superiorly and on either side by the left atrium, pulmonary veins, and inferior vena cava. The esophagus is related posteriorly to this space.
The second space of note within the pericardial sac is the transverse sinus. This is a potential space behind the pulmonary artery and ascending aorta. It is bounded from behind by the front of the atria and the superior vena cava. The surgeon can place a digit or two, or a ligature, into this space without dissection, and quickly clamp the great arteries.
The transverse sinus is separated from the oblique sinus by the venous mesocardial reflection. The venous mesocardial reflection runs from the pericardial sac to the dorsum of the left atrium between the uppermost right and left pulmonary veins. From a clinical standpoint, the pericardium should be considered a single entity, a closed fibroserous sac (see Fig. 7-3).
In the cadaver, the pericardial cavity contains between 40 and 60 ml of fluid. Much more can be accommodated if the increase in quantity is gradual.
RELATIONS OF THE PARIETAL PERICARDIUM
The pericardial sac is roughly conical in shape. It is fused at its base to the diaphragm, and fused at its apex to the adventitia of the great vessels and pretracheal fascia. Two other minor points of fixation are the superior and inferior sternopericardial ligaments.
The relations of the pericardium are as follows:
|
Anterior: The fibrous pericardium is related to the sternum and the costal cartilages, but is separated from them, for the most part, by the anterior medial reflections of the left and right pleurae (the costomediastinal reflections). The pericardium is thus covered by the pleurae, except over a small bare area on the left at the level of the fourth to sixth cartilages. This is known as the “bare area of Edwards,” or the “cardiac dull space.” The latter term is attributable to the lack of resonance to percussion at this point. Posterior: The right and left bronchi, lymph nodes, esophagus and its nerve plexus, descending thoracic aorta, and vertebral reflection of pleura are all related to the posterior portion of the pericardium (Figs. 7-4, 7-5) Lateral: Mediastinal pleurae, phrenic nerves, and pericardiacophrenic vessels Inferior: Diaphragm, peritoneum, and inferior vena cava Superior: Roots of the great vessels, the left brachiocephalic vein, the left recurrent laryngeal nerve, and the left superior intercostal vein |
|
||
|
|
||
|
William Osler envisioned an “abdominal area of romance where the head of the pancreas lies folded in the arms of the duodenum.” We like to think that within the chest cavity also there is a love affair, with the lungs embracing the pericardial sac and the heart.
Vascular Supply
ARTERIES
About 80 percent of the blood to the pericardium comes from the right and left internal thoracic arteries by way of their pericardiacophrenic branches (Fig. 7-6). In addition, the lower pericardium is supplied by branches of the superior phrenic arteries. The posterior portion receives branches from the bronchial and esophageal arteries and mediastinal twigs from the descending thoracic aorta. All of these vessels anastomose freely.
|
||
|
VEINS
The veins follow the arteries. They empty into the azygos and hemiazygos veins, the internal thoracic veins, and the superior phrenic veins.
LYMPHATICS
The pericardium is drained by three groups of lymph nodes:
|
Anterior mediastinal nodes Diaphragmatic nodes Inferior tracheobronchial nodes |
Warren11 reported on pericardial malignancies:
Malignancies rarely arise from the pericardium. Mesothelioma, the most common of these, is usually unresectable and almost always incurable. Malignancies may secondarily involve the pericardium by direct extension…More frequently, malignancies may involve the pericardium by a process of retrograde lymphangitic spread or hematogenous dissemination. These patients present with a symptomatic pericardial effusion and occasionally pericardial tamponade. Subxiphoid pericardiostomy and drainage is a safe procedure that provides effective and durable symptomatic relief in these terminally ill patients.
Innervation
Nerve fibers from the vagus nerves, the phrenic nerves, and the cardiac branches of the recurrent laryngeal nerves supply the parietal pericardium. Sympathetic fibers arise from the cervical and upper thoracic parts of the sympathetic chains, and from the stellate ganglia. The fibers reach the pericardium by way of the aortic and cardiac plexuses.
Surgical Applications
PERICARDIOCENTESIS
Aspiration of the fluid contents of the pericardial sac may be necessary for the diagnosis or treatment of pericardial effusion caused by trauma, secondary manifestations of heart disease, infection, or neoplasms. Cardiac tamponade may also be produced from central venous catheters. Unexplained hypotension, tightness of the chest, and shortness of breath are the signs and symptoms of cardiac tamponade. Collier et al.12 advised emergency echocardiogram for diagnosis, but for prevention advised that the tip of the catheter should be outside of the cardiac silhouette on chest X-rays. It is obvious that the greater the accumulated amount of fluid, the easier the aspiration, but the more desperate the patient’s condition.
Remember Beck’s Triad of cardiac tamponade:
|
Small, quiet heart Falling atrial pressure Rising venous pressure |
We quote from Schrump and Nguyen13 on malignant pericardial effusion:
Malignant pericardial effusion is frequently an indication of advanced, incurable malignancy. Hence, the goals of intervention include relief of symptoms and prevention of recurrence…Surgical interventions (subxiphoid pericardiostomy) or medical interventions (ultrasound-guided percutaneous tube pericardiostomy and sclerotherapy) have acceptable risks and provide excellent results. We favor surgical drainage as the primary approach for patients with malignant pericardial effusion because of its simplicity and extremely high success rate without the need for intrapericardial instillation of sclerosing agents and tube manipulations that may be associated with patient discomfort. Recurrent malignant pericardial effusion can be managed either by repeat pericardiostomy or insertion of a shunt. Patients responding to treatment with complete control of the effusion should have a meaningful survival with life expectancy (average, 9 months) contingent on the histology of the underlying malignancy.
Parasternal Approach
The needle is inserted into the fifth or sixth intercostal space 2 cm lateral to the apical impulse, or just medial to the left border of the cardiac dullness. The needle is then directed to the right shoulder. The parasternal position of the internal thoracic artery and vein must be remembered to avoid hemothorax from their laceration.
Abdominal (Paraxiphoid) Approach
The needle is inserted 1 cm below and 1 cm to the left of the xiphoid, between it and the left costal arch, pointing in the direction of the left shoulder. This is the preferable route, because the needle will transgress neither the pleural nor the peritoneal cavities; most importantly, it is less likely to cause injury to a coronary artery.
Olsen et al.14 recommended pericardial-peritoneal window for patients with malignant and non-infectious benign pericardial effusions, including those with tamponade.
PERICARDIOTOMY AND PERICARDIECTOMY
Indications include constrictive pericarditis, and malignant or benign constrictive effusion.
Approaches
For the drainage of the pericardial space and/or partial pericardiectomy, two approaches may be used, the subxiphoid and the anterolateral.
For the subxiphoid approach, a midline incision is made from approximately the xiphoid process to approximately halfway above the umbilicus. The xiphoid process is then resected. With downward traction of the diaphragm, the anterior pericardium is exposed and resected.
For the anterolateral approach, an anterolateral thoracotomy at the left fifth intercostal space is made, and part or all of the left anterolateral pericardium is removed.
For total pericardiectomy, median sternotomy is the best approach, although the “clamshell” bilateral submammary incision may be used in special cases. The pericardium is removed from the aorta and pulmonary artery above to the diaphragm below, and from the left to the right pulmonary veins.
Inflammatory Response to Pericardial Trauma
In some patients, opening of the pericardium may be accompanied by fever, pericardial and pleural effusion, and/or pain. These manifestations have been termed ‘postpericardiotomy syndrome.’ Injury to the pericardium and the presence of blood in the pericardial cavity appears to be the cause. Salicylates and corticosteroids provide relief from the symptoms. The condition is usually self-limiting.
Heart
External Topographic Features
PROJECTION OF THE HEART ON THE ANTERIOR CHEST WALL
The projection of the living heart on the chest wall (Fig. 7-7) is highly variable. It depends upon the position of the body and other factors such as age and obesity. There are four anatomic landmarks, identified in Figure 7-7 by Roman numerals I-IV:
|
|
||
|
If you connect the four Roman numerals as indicated below, the figure so outlined (Fig. 7-7) provides a rough approximation of the projection of the heart. This projection can never be taken for granted, because the heart is not rigidly fixed in the thorax:
|
I and II with a convex line (SVC to IVC) II and III with a straight line (IVC to apex) III and IV with a convex line (apex to tip of left auricle) IV and I with a straight line (tip of left auricle to SVC) |
The projection of the four cardiac valves (Fig. 7-8) is approximately as follows:
|
P, Pulmonary valve: Third left sternochondral junction A, Aortic valve: Left sternal line at third left intercostal space, just below and medial to the pulmonary valve projection M, Mitral valve: Fourth left sternochondral junction T, Tricuspid valve: Right sternal line at fourth left intercostal space |
|
||
|
The location of the points of best auscultation of these valves is different from their actual projections. Valve sounds are best heard at the following sites (Fig. 7-8):
|
P, Pulmonary valve: Second left intercostal space, adjacent to the sternum A, Aortic valve: Second right intercostal space, adjacent to the sternum M, Mitral valve: Fourth or fifth left intercostal space, near the midclavicular line (apex beat) T, Tricuspid valve: Fourth or fifth left sternochondral junction, near the end of the sternum (right lower sternal line) |
According to Waller and Schlant,15 the weight and size of the heart vary, depending on such factors as age, sex, body length, epicardial fat, and general nutrition. Edwards16 stated that the adult human heart averages  325 ± 75 g in men and 275 ± 75 g in women.
325 ± 75 g in men and 275 ± 75 g in women.
Anterior or Sternocostal Surface
The right atrium and auricle, the atrioventricular groove, and the right ventricle and pulmonary outflow tract, or conus arteriosus, form the anterior surface of the heart. The anterior right ventricle is typically in nearly direct contact with the sternum. Occasionally, a small portion of the left ventricle participates in the formation of the anterior surface (Figs. 7-9, 7-10).
|
||
|
|
||
|
Remember
|
With median sternotomy, the atrial appendages in a normal heart are located clasping the arterial pedicle (Fig. 7-11) in most cases. If the atrial appendages are on the same side of the pedicle, they produce an anomaly known as juxtaposition of the appendages. This anomaly can also be associated with congenital heart disease. |
|
||
|
Posterior Surface
The posterior surface of the heart consists of the left ventricle, the atrioventricular and posterior interventricular sulci, the left atrium and its four (or five) pulmonary veins, and a portion of the right atrium (Figs. 7-12, 7-13).
|
||
|
|
||
|